PRRS virus has a strongly restricted tropism for some sub-populations of macrophages found in lungs, placenta and lymphoid organs.
Recent studies point out that PRRS virus is also able to infect and replicate in other subtype of cells, such as dendritic cells derived either from bone marrow or from monocyte cells. Conversely, monocytes, peritoneal macrophages, bone marrow progenitor cells and pulmonary and plasmacytoid dendritic cells are resistant to viral infection.
Six potential cellular receptors for PRRS virus –heparan sulphate, vimentin, CD151, CD163, porcine sialoadhesin (PoSn or CD169) and DC-SIGN (CD209)- have been described. Out of them all, only PoSN and CD163 are exclusively expressed in macrophages.
To date, CD163 has been determined to be the major receptor that mediates viral internalization and disassembly. As a matter of fact, it has been demonstrated that genetically edited pigs lacking CD163 are resistant against PRRS virus infection.
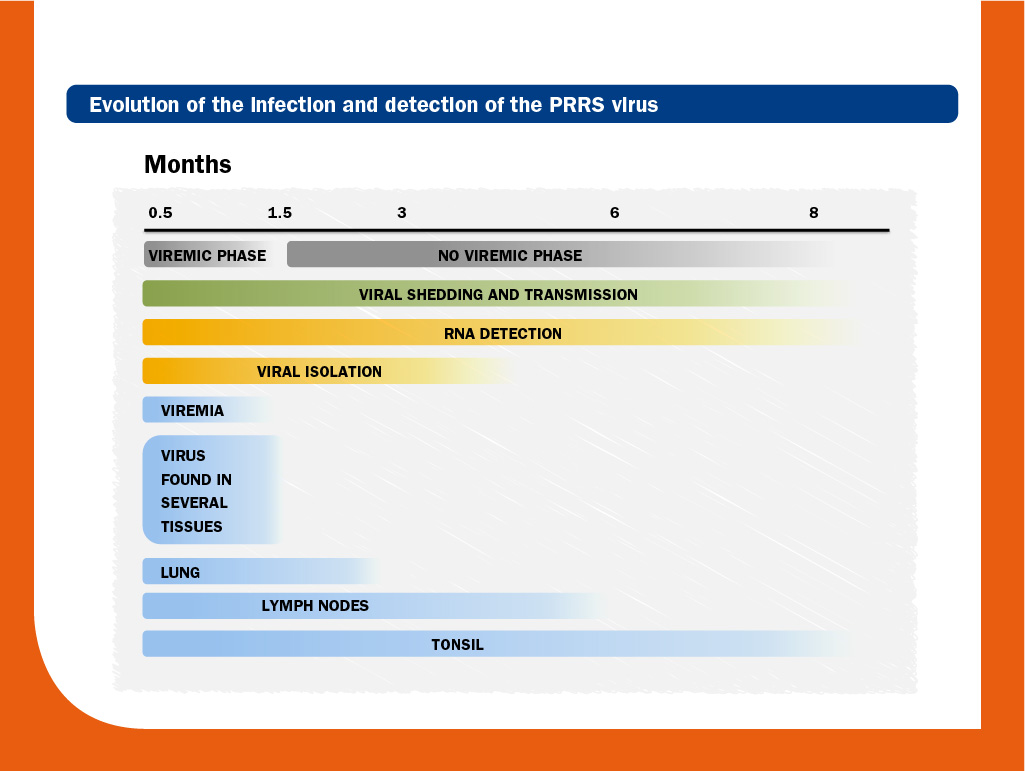
Initially, virus replication occurs in local permissive macrophages, predominantly in lungs. Virus can be detected in blood (viraemia) within hours (6-12h) and viral load peaks around one week post-infection. The length of viraemia is strongly influenced by the strain and the animal’s age. Thus, viraemia may extend for several weeks in piglets, whereas in adult pigs may last for a few days only.
The virus spreads to lymphoid organs, such as the tonsils and lymph nodes, and less consistently, to other tissues, by the lympho-hematic way. After the viraemic phase, the virus is confined for long time in secondary lymphoid tissues at low viral replication; in some cases, antigen has been detected until day 251 and infectious virus could be isolated until day 157.
Moreover, PRRS virus from congenitally infected piglets can be transmitted to sentinel animals until 112 days after birth. Mechanisms of persistence have not been clearly identified yet, but it has been suggested that selection within viral subpopulations/quasispecies and immune response can play a role.
In lungs, the predominant cells supporting PRRS virus replication are pulmonary alveolar macrophages and pulmonary intravascular macrophages. The virus compromises their functions and provokes an inflammatory cell infiltration resulting in interstitial pneumonia which, in conjunction with enlarged lymph nodes, is a common lesion in a PRRS virus infection. In this regard, it is important to note that interstitial pneumonia is nonspecific for PRRS virus.
Clinical disease and lesions due to PRRS virus infection may occur by a variety of mechanisms that at least include: apoptosis of infected and non-infected macrophages, induction of polyclonal B-cell activation and induction of inflammatory cytokines.
PRRS virus can cause more complex diseases when it acts as primary agent in porcine respiratory disease complex (PRDC). In clinical cases of PRDC, PRRS virus is the most common virus isolated. There are several evidences of its interaction with other viruses and bacteria.
It is important to point out that different isolates replicate with different intensity in different tissues. This fact could have important implications for intensity of replication, virulence and transmission.
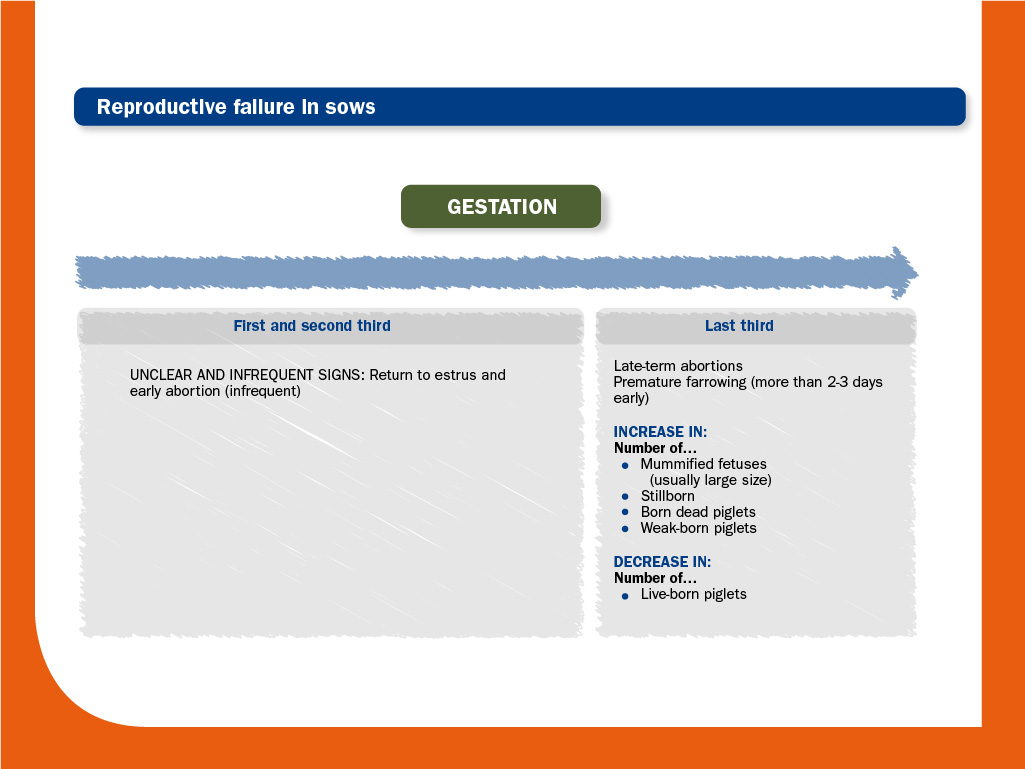
Regarding reproductive disorders, the severity of clinical manifestations due to PRRS virus infection increases as the gestation progresses, being return to estrus or early abortion very rare.
The number of PRRS virus receptors in fetal placenta and fetal macrophages permissiveness to the virus increase over time.
Moreover, the virus crosses the placental barrier most efficiently in late gestation (after day 85, approximately) by infected macrophages. Then, fetuses can be infected directly by the sow or alternatively, by other infected fetus.
In the past, it was suggested that fetal death results from events occurring at the maternal-fetal interface and that was independent of direct fetal involvement; however, recent studies have demonstrated that viral replication in fetal tissues and subsequent spread to adjacent fetuses are crucial events in the pathogenesis.
As a result, PRRS virus can cause abortion or premature birth affecting the viability of a variable percentage of the fetuses.
Clinical presentation greatly varies due to the virulence of PRRS virus strains, the level of immunity, the age of the pigs and the reproduction stage of the sows and the coexistence of other pathogens, among others.
An outbreak usually begins in more than one stage of production and during two weeks it is characterised by anorexia, fever and lethargy in animals of different ages. In the breeding herd, most sows usually abort from 3 to 21 days post-infection. This picture may last for months.
The following list shows a simplified summary of the clinical signs. Obviously, the full list of clinical signs does not appear in all infected animals. Moreover, during an outbreak, not all infected animals show clinical signs.
Respiratory clinical signs:
- Null or low mortality, except for severe cases.
From June to September 2006, an atypical form of PRRS emerged in China affecting over two million pigs, of which 400,000 died. Since then, the highly-pathogenic PRRS virus (HP-PRRSV) strains have spread throughout Asia. Unlike previous PRRS outbreaks, this form of PRRS virus was more virulent and should be considered a totally separate entity.
The range of symptoms seems to be wider, affecting kidneys, liver and spleen and the disease can be far more severe, with reported abortion rates of up to 50% and mortality rates of over 10% in adult pigs.
© Laboratorios Hipra, S.A. 2024. All Rights Reserved.
No part of this website or any of its contents may be reproduced, copied, modified or adapted, without the prior written consent of HIPRA.