Passive transfer studies have demonstrated that specific antibodies against PRRS virus are capable of protecting animals from homologous challenge.
However, the role of NAs in the course of infection is a controversial issue.
While some authors have reported a certain correlation between the appearance of NAs and the cease of viraemia or the clearance of the virus from lungs, other studies have suggested that for some strains, clearance of viraemia can occur without NAs; even more, viraemia and viral replication can persist even in the presence of NAs.
In relation to cell-mediated immunity, some studies have reported that IFN-γ may be associated with viral clearance in the absence of NAs.
Protection against re-infection:
As has been widely discussed, levels of NAs after PRRS virus infection/vaccination are usually low or nil.
Indeed, NAs effectiveness is limited against heterologous isolates since cross-neutralisation (neutralisation of a given strain by NAs induced by another strain) can be low even between similar strains.
It seems that cross-neutralisation may not be strictly related to the sequences and/or number of glycosylations of known neutralising epitopes in GP3, GP4 or GP5; this has led some authors to claim that other, yet unknown, neutralising epitopes could exist.
Nevertheless, although exceptions exist, it seems that the higher the amount of NAs, the better protection against re-infection.
In terms of cell-mediated immunity, several studies have related higher frequencies of IFN-γ-SC, in the absence of NAs with clinical and virological protection.
This phenomenon has been described in sows and in piglets in terms of protection against reproductive and respiratory failures, respectively.
However, it is important to point out that as seen for the NAs, resolution of viraemia can be achieved even without apparent development of IFN-γ-SC.
Some studies have demonstrated that pigs with low IFN-γ-SC responses after vaccination can be protected from a PRRS virus challenge if high levels of NAs are present.
Examples of the role of NAs and cell-mediated immunity in viral clearance and protection:
- Example I. Cell-meditated immunity and neutralising antibodies in vaccinated piglets:
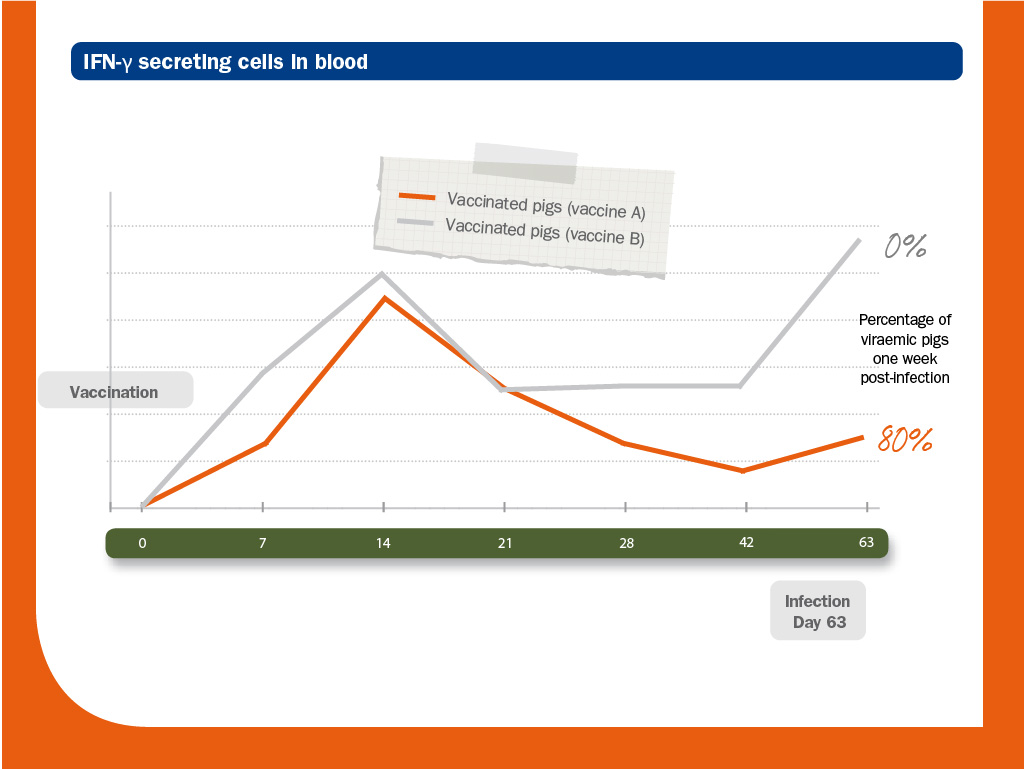
Two groups of piglets were vaccinated (vaccine A and B) and challenged (Ch strain).
As expected, vaccinated piglets did not develop NAs.
In this situation, IFN-γ-SC was most likely the main protecting mechanism against infection.
Genetic identity between the structural proteins of the vaccines (A and B) and the strain used in the challenge (Ch) was above 90%; however, it was higher between vaccine A and Ch (98.3-99.7%) than between vaccine B and Ch (92.3-98.6%).
Interestingly, in this case, heterologous strain (B) provided better protection than the homologous one (A). According to the authors, this fact could be explained by the ability of each vaccine to induce PRRS virus-specific IFN-γ-SC. Source: Díaz et al. (2006).
- Example II. Cell-mediated immunity in vaccinated piglets (field conditions):
Vaccinated piglets were not completely protected against infection, but the disease had a less severe course.
Interestingly, higher clinical scores and viraemia were observed in low PRRS virus-specific IFN-γ-SC responders. Source: Martelli et al. (2009). - Example III. Cell-meditated immunity and neutralising antibodies in homologous and heterologous challenges:
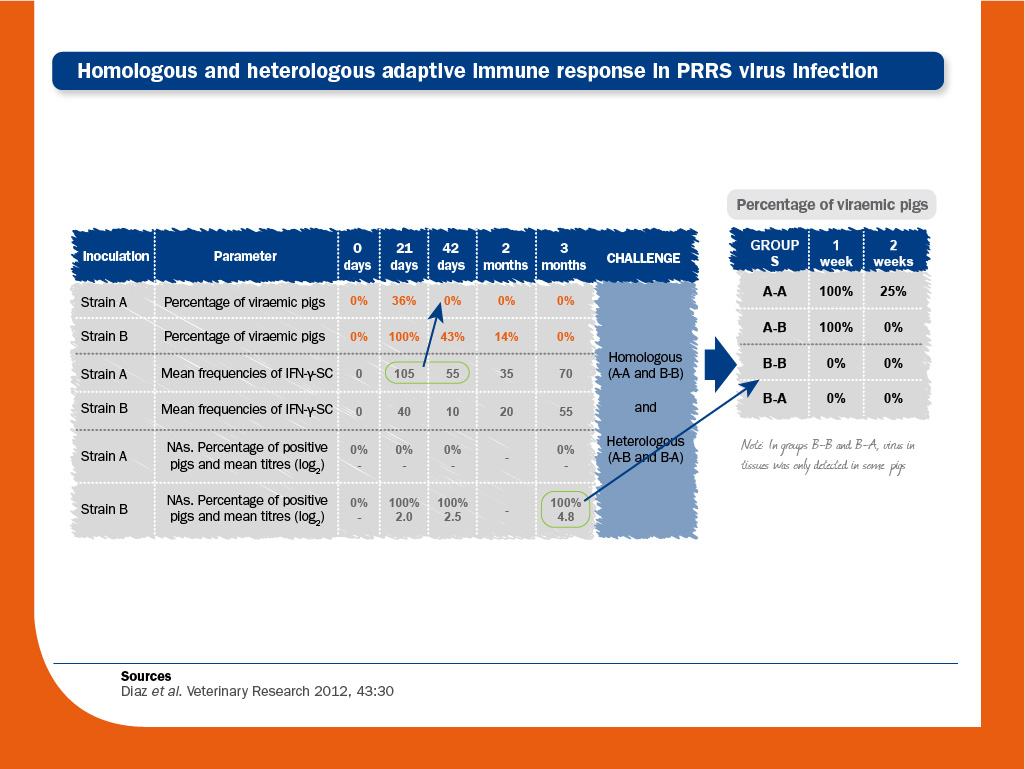
In order to investigate the significance of each compartment in protection against PRRS virus, the homologous and heterologous immune responses in PRRS virus-infected piglets were characterised.
One group of animals were first inoculated with PRRS virus strain A and three months later they were challenged with either strain A or strain B (A-A and A-B) and followed-up for the next two weeks.
Another group of pigs were inoculated with strain B and challenged at three months as above (B-B and B-A).
The main results and conclusions of this study were as follows:
- Viral clearance after infection. In animals inoculated with strain A, viraemia ceased in the absence of NAs. In animals inoculated with strain B, viraemia was longer and sustained, and coexisted for weeks with NAs.
Therefore, the role of NAs in clearing viraemia could be considered as limited. Regarding cell-mediated immunity, strain A induced the highest frequencies of PRRS virus-specific IFN-γ-SC.
Since NAs were not detected in this group of animals, it would indicate that PRRS virus-specific IFN-γ-SC was responsible for the viral clearance. - Protection against re-infecion. After re-infection, virus was not detected in blood from animals previously inoculated with strain B (B-B and B-A).
Some of them were actually infected, since virus could be detected in tissues after either homologous (B-B) or heterologous challenge (B-A).
However, infection in these pigs was at lower intensity than in A-A and A-B animals.
Therefore, as opposed to viral clearance, preformed NAs play a role in protection against re-infection.
Again, since strain A did not induce NAs production, but induced the highest frequencies of PRRS virus-specific IFN-γ-SC, it would indicate that cell-meditated immunity could be responsible for limiting the duration of viraemia and the spread of the virus to the tissues when NAs are not present.
Overall, the results of this study suggested that “preformed NAs may have a protective role against infection, but animals devoid of detectable NAs may limit the spread of infection, thus indicating that cell-mediated immunity probably also contributes to protection”.
© Laboratorios Hipra, S.A. 2024. All Rights Reserved.
No part of this website or any of its contents may be reproduced, copied, modified or adapted, without the prior written consent of HIPRA.
- Bautista EM, Molitor TW. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997, 10: 83-94.
- Bautista EM, Molitor TW. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch Virol. 1999, 144:1191-200.
- Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI. Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic. Immunol Res. 2014, 59:81-108.
- Darwich L, Díaz I, Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Res. 2010, 154:123-32.
- Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006, 351:249-59.
- Díaz I, Gimeno M, Darwich L, Navarro N, Kuzemtseva L, López S, Galindo I, Segalés J, Martín M, Pujols J, Mateu E. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Vet Res. 2012, 19:43:30.
- Dwivedi V, Manickam C, Binjawadagi B, Linhares D, Murtaugh MP, Renukaradhya GJ. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol J. 2012, 9:45.
- Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009, 27:3704-18.
- Labarque GG, Nauwynck HJ, Van Reeth K, Pensaert MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000, 81:1327-34.
- Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004, 102:155-63.
- Lowe JE, Husmann R, Firkins LD, Zuckermann FA, Goldberg TL. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J Am Vet Med Assoc. 2005, 226:1707-11.
- Lunney JK, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154:1-6.
- Lunney JK, Fang Y, Ladinig A, Chen N, Li Y, Rowland B, Renukaradhya GJ. Porcine reproductive and respiratory syndrome virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu Rev Anim Biosci. 2016, 4:129-54
- Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, Bottarelli E, Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009, 27:3788-99.
- Martínez-Lobo FJ, Díez-Fuertes F, Simarro I, Castro JM, Prieto C. Porcine Reproductive and Respiratory Syndrome Virus isolates differ in their susceptibility to neutralization. Vaccine. 2011, 29:6928-40.
- Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008, 177:345-51.
- Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003, 309:18-31.
- Mengeling WL, Lager KM, Vorwald AC, Koehler KJ. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2003, 93:13-24.
- Miranda J, Torrents D, Busquet M, Fenech M, Mateu E, Díaz I. Heterologous cell-mediated immune responses against PRRS virus in gilts vaccinated intramuscularly and intradermally with UNISTRAIN® PRRS. 2015, 7th International Symposium on Emerging and Re-emerging Pig Diseases.
- Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002, 15:533-47.
- Murtaugh MP, Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine. 2011, 29:8192-204.
- Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154:18-30.
- Rose N, Renson P, Andraud M, Paboeuf F, Le Potier MF, Bourry O. Porcine reproductive and respiratory syndrome virus (PRRSv) modified-live vaccine reduces virus transmission in experimental conditions. Vaccine. 2015, 33:2493-9.
- Pileri E, Gibert E, Soldevila F, García-Saenz A, Pujols J, Diaz I, Darwich L, Casal J, Martín M, Mateu E. Vaccination with a genotype 1 modified live vaccine against porcine reproductive and respiratory syndrome virus significantly reduces viremia, viral shedding and transmission of the virus in a quasi-natural experimental model. Vet Microbiol. 2015, 175:7-16.
- Prieto C, Alvarez E, Martínez-Lobo FJ, Simarro I, Castro JM. Similarity of European porcine reproductive and respiratory syndrome virus strains to vaccine strain is not necessarily predictive of the degree of protective immunity conferred. Vet J. 2008, 175:356-63.
- Roca M, Gimeno M, Bruguera S, Segalés J, Díaz I, Galindo-Cardiel IJ, Martínez E, Darwich L, Fang Y, Maldonado J, March R, Mateu E. Effects of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. 2012, 193:92-6.
- Royaee AR, Husmann RJ, Dawson HD, Calzada-Nova G, Schnitzlein WM, Zuckermann FA, Lunney JK. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol. 2004, 102:199-216.
- Scortti M, Prieto C, Martínez-Lobo FJ, Simarro I, Castro JM. Effects of two commercial European modified-live vaccines against porcine reproductive and respiratory syndrome viruses in pregnant gilts. Vet J. 2006, 172:506-14.
- Weesendorp E, Morgan S, Stockhofe-Zurwieden N, Popma-De Graaf DJ, Graham SP, Rebel JM. Comparative analysis of immune responses following experimental infection of pigs with European porcine reproductive and respiratory syndrome virus strains of differing virulence. Vet Microbiol. 2013, 163:1-12.
- Yoon KJ, Wu LL, Zimmerman JJ, Platt KB. Field isolates of porcine reproductive and respiratory syndrome virus (PRRSV) vary in their susceptibility to antibody dependent enhancement (ADE) of infection. Vet Microbiol. 1997, 55:277-87.
- Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: 10th ed. Diseases of swine, Ed. Wiley-Blackwell. 2012, 31:463-86.